Video on contract manufacturing
for pharmaceuticals
"Welcome to eurimsolutions!" Our production film outlines the steps that we implement for you as a service provider on the basis of the manufacturer's license according to the legal requirements. The process includes the packaging of pharmaceuticals in the range of standard goods, chilled goods between 2°C – 8°C, controlled substances and medical devices according to the Counterfeit Protection Directive for Pharmaceuticals (FMD).
Perfect handling from A to Z
- The entire handling of the goods complies with the Counterfeit Protection Directive for Pharmaceuticals (FMD).
- This means that all steps in the process comply with all safety standards.
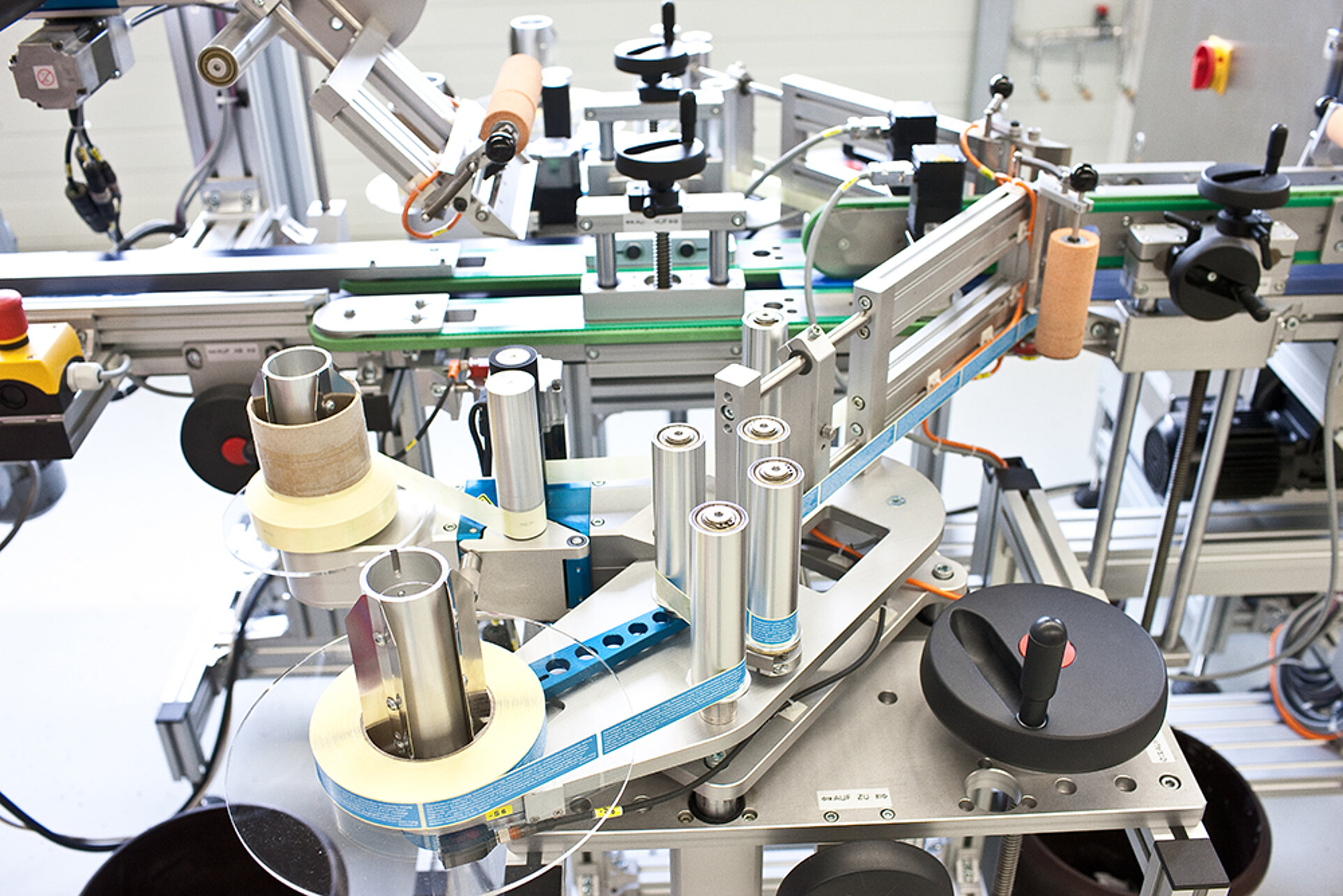
- Each individual process, such as sorting the drugs by product code, is automated and highly efficient at eurimsolutions.
- We serialise the medicine boxes, seal the product/medicines and upload the unique identification to the European hub.
The European hub is the European Medicines Verification System (EMVS).
Printing from a single source
- In our in-house print shop, we can also produce small batches of folding boxes for medicines, including printing of batch data using thermal ink jet technology.
- The corresponding package inserts for the drugs are produced just in time to match the folding boxes.
- Whether small or large batches – eurimsolutions offers the perfect tailor-made solution for your order.
Labelling, storage and shipping
- The marking of blister packaging, the labelling of folding boxes and the monitoring of serialisation data are highly automated in our production. The serialisation data is checked automatically for the legibility of plain text and 2D codes.
- Depending on the type of drug, the goods are always stored in accordance with the legal requirements.
- We are also happy to commission your orders directly at our premises using our logistics system. Here, the individual folding boxes with the pharmaceuticals are picked via Pick by Light and then sent to your customer at a controlled temperature.
We guarantee you the highest safety and processing standards in all work steps. GMP and GDP certifications have tested and confirmed these qualities.
The services of eurimsolutions also fit your order!
We look forward to hearing from you!